Newsletters
Electronic Data Capture (EDC)

The promise of electronic data capture (EDC) to improve the quality, speed and cost of clinical trials is too significant to be ignored. Faced with challenges such as greater competition, increasing time-to-market pressure and budgetary limitations, choosing to carry out trials on an electronic web-based EDC platform is a solution that dramatically improves your clinical trial’s performance. An EDC solution makes the task of entering clinical data as easy as possible and allows data to accessible anywhere you have an internet connection. EDC is built to put control of trial information in the sponsor’s hands. It is a customer-oriented tool that provides the sponsor with total access to trial information, thereby supporting decisions based on real-time information. Here are just a few ways in which using EDC shortens timelines, improves the quality of data and helps to cut trial costs.
Reduced Timelines:
The EDC shortens the timelines from monitoring to data cleaning and database lock. Described below is the shortened CRF Processing on EDC in compare to paper CRF:
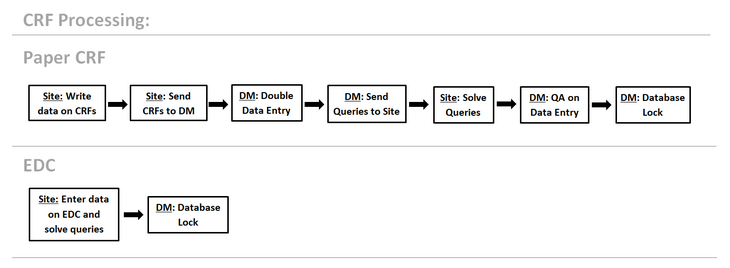
Using EDC cuts out a number of steps and data processing and brings the digital database closer to the original source data allowing for cleaner data and more accurate results. Specifically:
- Since the site enters the data directly into the EDC system and fewer transfers of data are required, fewer errors are entered into the database.
- Instantaneous pop-up queries encourage site staff to make sure the data is entered correctly at the moment it is entered, while the information is still fresh (instead of corrections being based on recalling events from months before).
- For complicated studies with many adverse events (AEs), medications or laboratory tests this important data can if written on paper pages. The EDC prevents that.
- The EDC forms are clean, centrally organized and no data gets lost.
- An audit trail is kept and every change made on the CRF is recorded, along with the reason for the change and who made the change.
- All data entered is legible! EDC ensures maximum data security as all entries to the EDC system require user name and password.
Enhanced Data Quality:
Using EDC cuts out a number of steps and data processing and brings the digital database closer to the original source data allowing for cleaner data and more accurate results.
Specifically:
- Since the site enters the data directly into the EDC system and fewer transfers of data are required, fewer errors are entered into the database.
- Instantaneous pop-up queries encourage site staff to make sure the data is entered correctly at the moment it is entered, while the information is still fresh (instead of corrections being based on recalling events from months before).
- Immediate entry of data into the EDC system means that in complicated studies with many adverse events (AEs), medications or laboratory tests this important data cannot be forgotten or lost on papers at the site.
- The EDC forms are clean, centrally organized and no data gets lost.
- An audit trail is kept and every change made on the CRF is recorded, along with the reason for the change and who made the change.
- All data entered is legible! EDC ensures maximum data security as all entries to the EDC system require user name and password.
Lower Study Costs:
In addition to the savings of saved time and higher data quality, EDC can bring down study costs in numerous other ways including:
- Fewer and shorter monitoring visits can be a source of dramatic savings.
- No need to print CRFs.
- No costs for processing of data clarifications (queries) since it is all done automatically in the EDC system.
- Overall, when bearing in mind cost-benefit calculations it makes a good business practice to embrace EDC.

We make it Professional - contact us
Address: 17 Masada Street, 12th Floor ,Bnei Brak, 5120118 . Israel
Tel: +972-9-7669333
Fax: +972-9-7660443
Email: info@technostat.co.il

